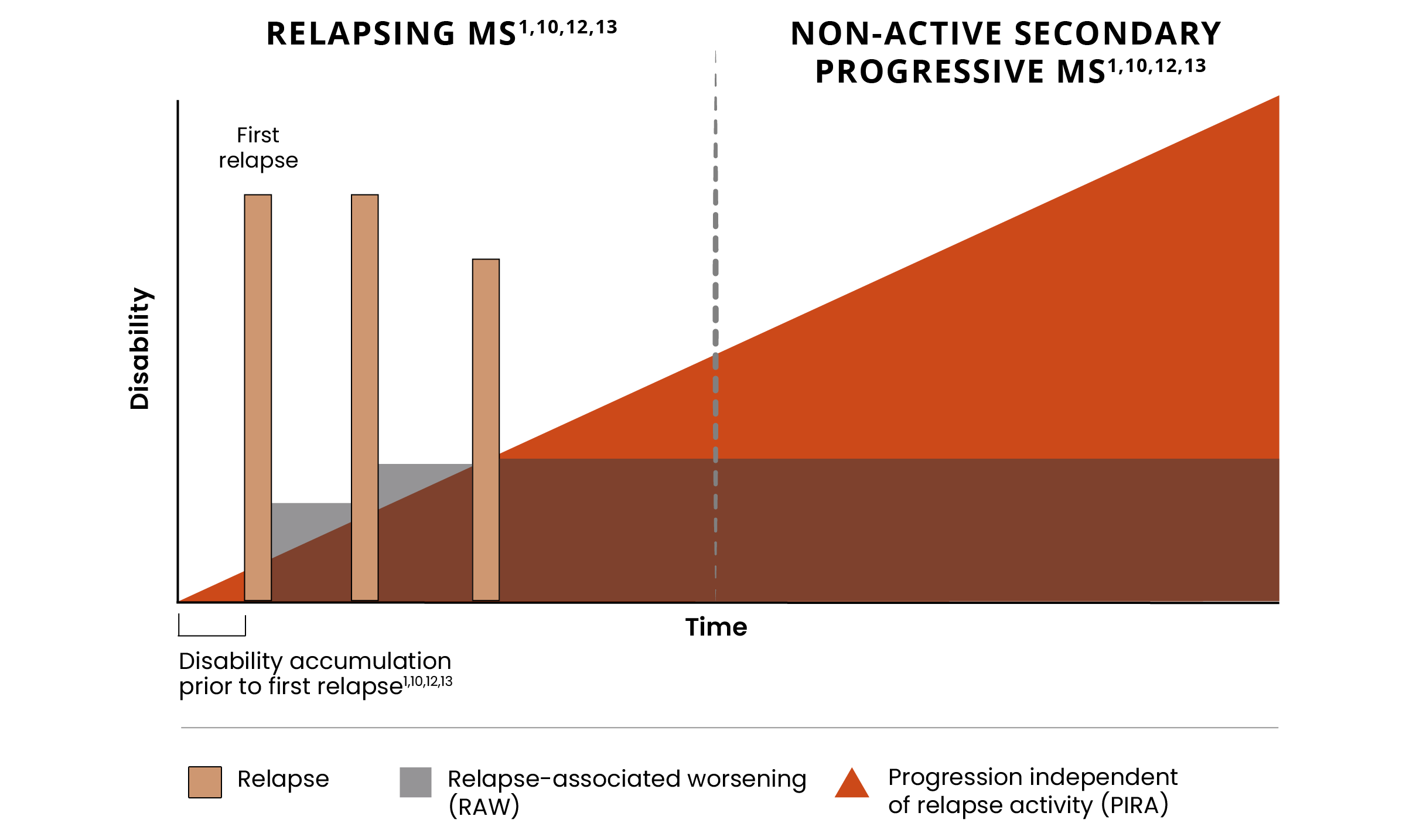
Addressing disability remains a significant unmet need across the MS spectrum1-3
Despite effective control of relapses and acute lesions, many people with MS continue to accumulate disability.1,4
Progression independent of relapse activity (PIRA) is an established phenomenon in relapsing-remitting multiple sclerosis (RRMS).5-11*†
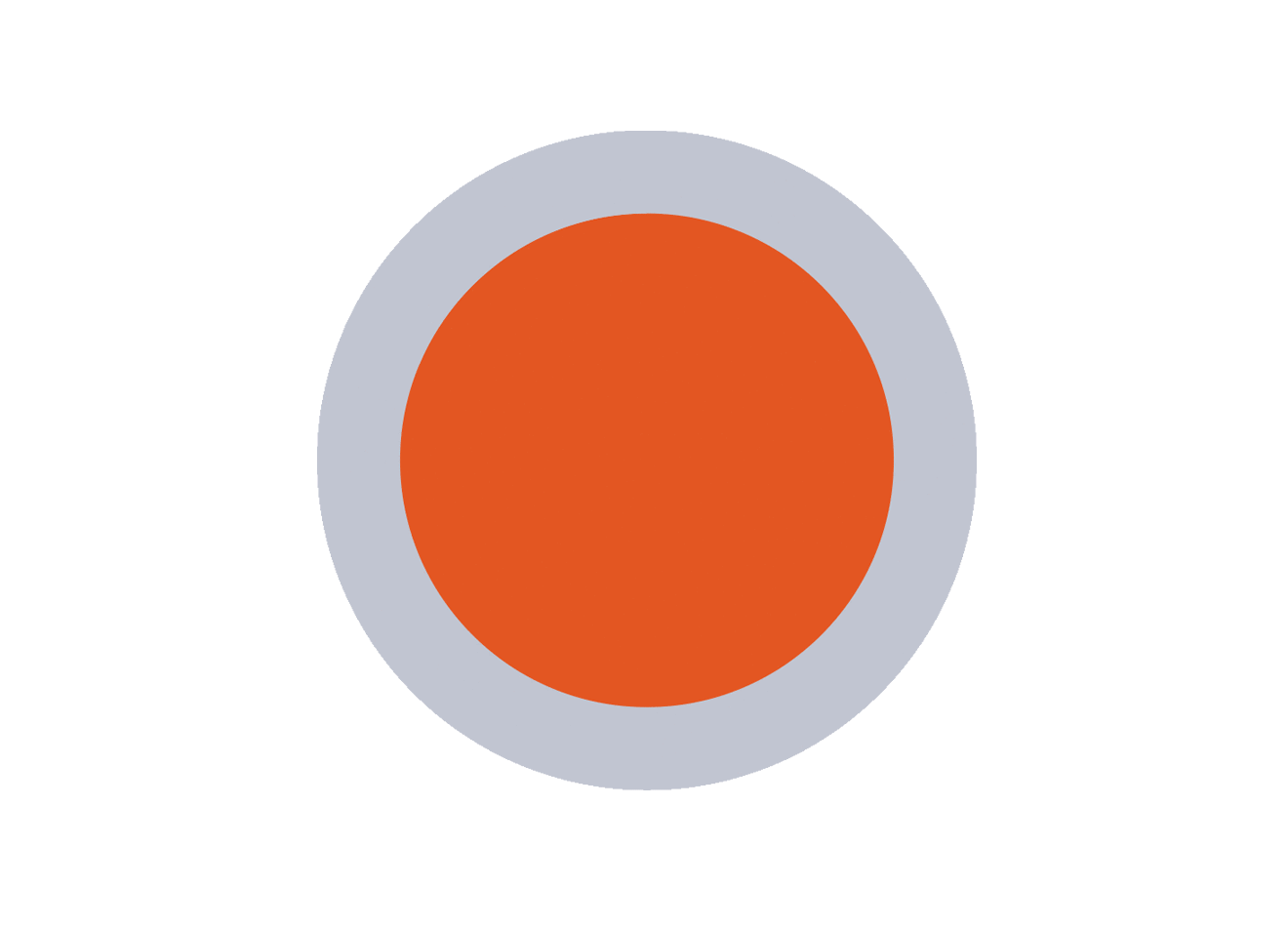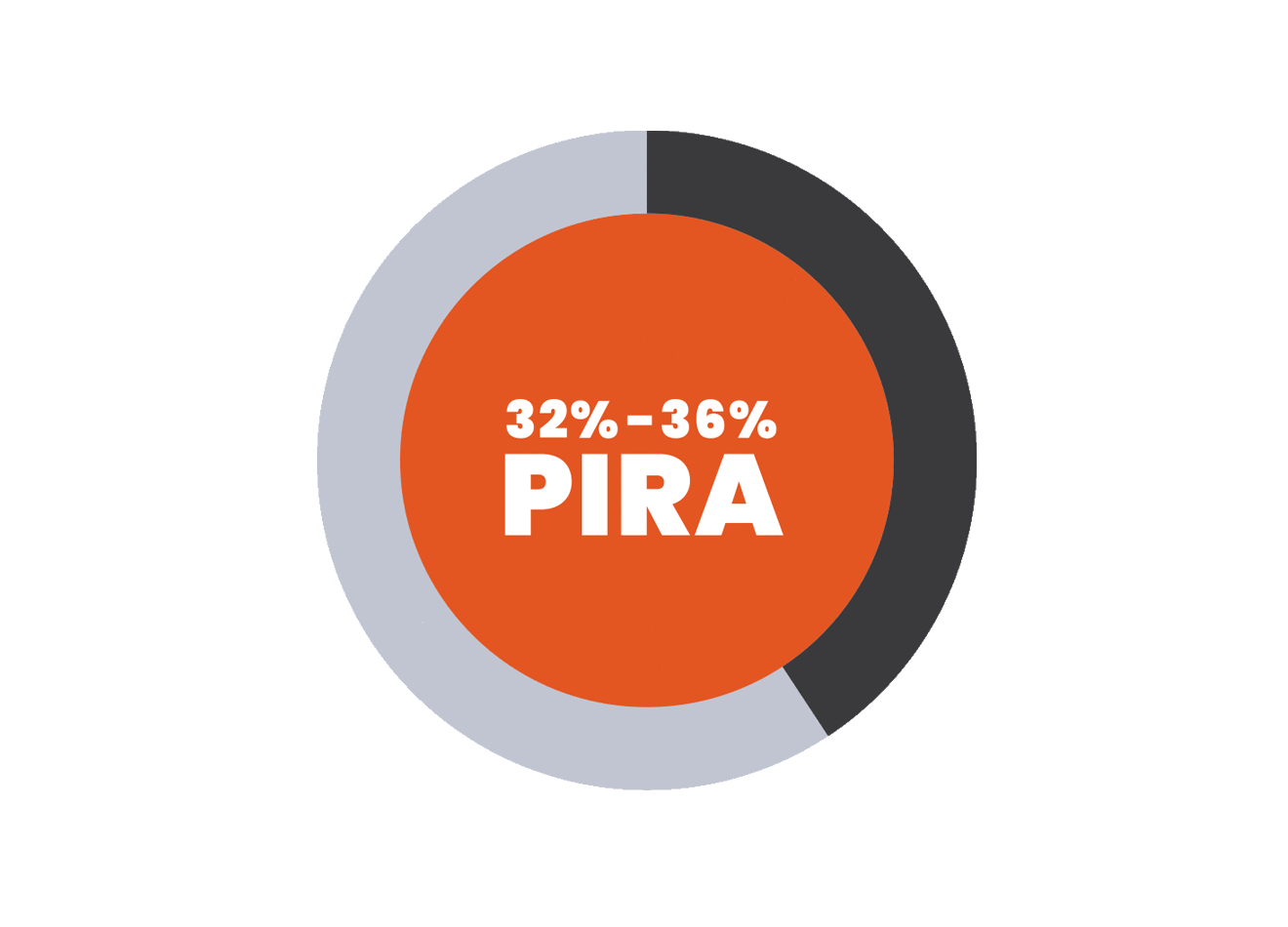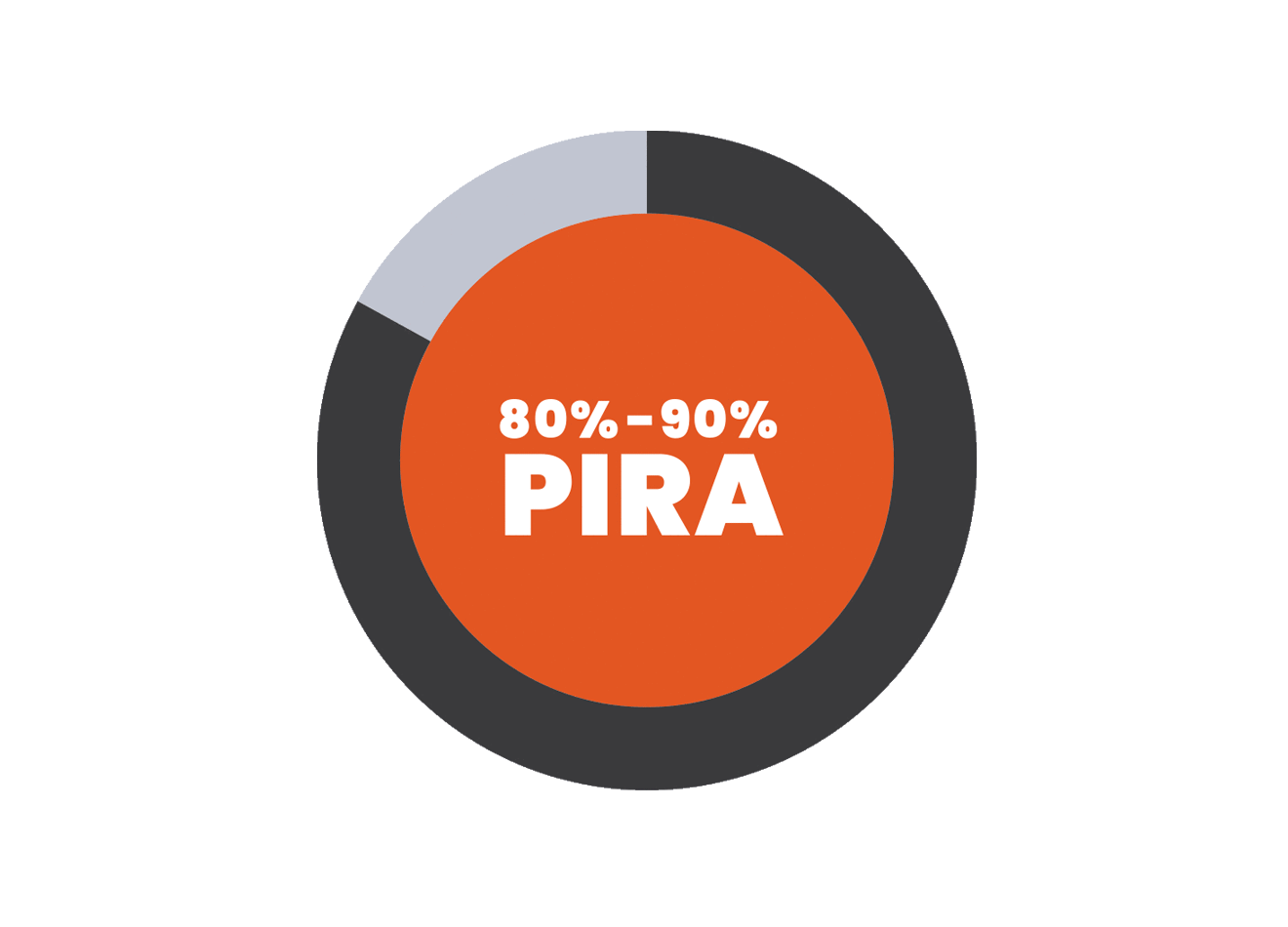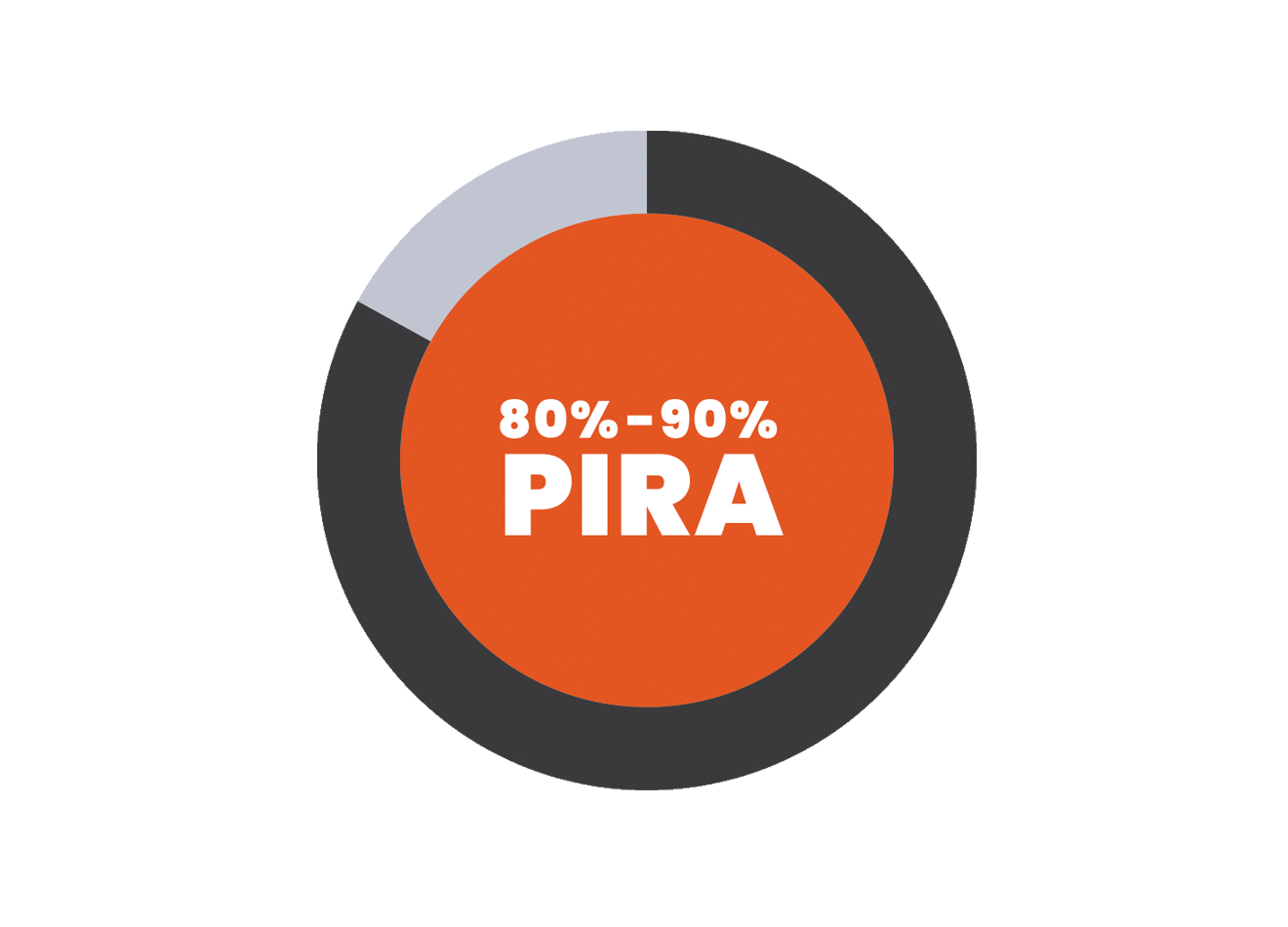
PIRA is responsible for up to 80% to 90% of disability accumulation in treated RRMS patients7,8
PIRA can be seen in treated RRMS patients as early as 3 years after diagnosis8-10
65% of RRMS patients reported experiencing PIRA5,6
Approximately 30%-40% of treated patients will transition to SPMS within 12 years of RRMS diagnosis11
*As documented by an observational study and pooled data from 2 randomized clinical trials.
†As seen in real-world data (patient-reported outcomes). PIRA was defined as continuous worsening of symptoms independent of relapses in the previous 12 months.
Addressing relapse-independent mechanisms of progression remains an unmet need across the disease spectrum from RRMS to non-active secondary progressive multiple sclerosis (naSPMS).1-3

Hear from the experts
Jeffrey Dunn, MD, discusses what drives disability in MS at ACTRIMS Forum 2024
The relapse-independent process remains unaddressed1,4,12
Many patients still progress from RRMS to secondary progressive multiple sclerosis (SPMS) and accumulate significant disability despite current treatments that effectively address relapses and acute lesions.1,4,12

Hear from the experts
Massimo Filippi, MD, FEAN, FAAN, discusses disability accumulation and PIRA at ECTRIMS 2023
Keep Exploring
See how smoldering neuroinflammation impacts disability accumulation
References:
- Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi:10.1177/17562864211066751
- Geladaris A, Torke S, Weber MS. Bruton’s Tyrosine Kinase inhibitors in multiple sclerosis: pioneering the path towards treatment of progression? CNS Drugs. 2022;36(10):1019-1030.
- Absinta M, Lassmann H, Trapp BD. Mechanisms underlying progression in multiple sclerosis. Curr Opin Neurol. 2020;33(3):277-285.
- Frisch ES, Pretzsch R, Weber MS. A milestone in multiple sclerosis therapy: monoclonal antibodies against CD20—yet progress continues. Neurotherapeutics. 2021;18(3):1602-1622.
- Müller J, Cagol A, Lorscheider J, et al. Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: a systematic review. JAMA Neurol. 2023;80(11):1232-1245.
- Bayas A, Schuh K, Christ M. Self-assessment of people with relapsing-remitting and progressive multiple sclerosis towards burden of disease, progression, and treatment utilization results of a large-scale cross-sectional online survey (MS Perspectives). Mult Scler Relat Disord. 2022;68:104166. doi:10.1016/j.msard.2022.104166
- Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140.
- Ingwersen J, Masanneck L, Pawlitzki M, et al. Real-world evidence of ocrelizumab-treated relapsing multiple sclerosis cohort shows changes in progression independent of relapse activity mirroring phase 3 trials. Sci Rep. 2023;13(1):15003. doi:10.1038/s41598-023-40940-w
- Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80(2):151-160. doi:10.1001/jamaneurol.2022.4655
- Krieger SC, Antoine A, Sumowski JF. EDSS 0 is not normal: multiple sclerosis disease burden below the clinical threshold. Mult Scler. 2022;28(14):2299-2303. doi:10.1177/13524585221108297
- Coret F, Pérez-Miralles FC, Gascón F, et al. Onset of secondary progressive multiple sclerosis is not influenced by current relapsing multiple sclerosis therapies. Mult Scler J Exp Transl Clin. 2018;4(2):2055217318783347. doi:10.1177/2055217318783347
- Filippi M, Amato MP, Centonze D, et al. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol. 2022;269(10):5382-5394.
- Giovannoni G. The neurodegenerative prodrome in multiple sclerosis. Lancet Neurol. 2017;16(6):413-414.